RESEARCH & TECHNOLOGY
Research Technologies
We Believe In
RESEARCH & TECHNOLOGY
Research Technologies
We Believe In
rHul L-12
The current immunotherapy modalities for OS 8 are:
non-specific
IL-12 immunomodulation
specific
monoclonal antibodies
antibody–drug conjugates
chimeric antigen receptor T cells targeting cell-surface proteins commonly overexpressed in OS
adoptive
im
PLGA
This is tab content. Click to edit this text. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
lL-12 + PLGA
This is tab content. Click to edit this text. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Diagnostic
This is tab content. Click to edit this text. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
rHulL-12
rHulL-12 Pre-Clinical
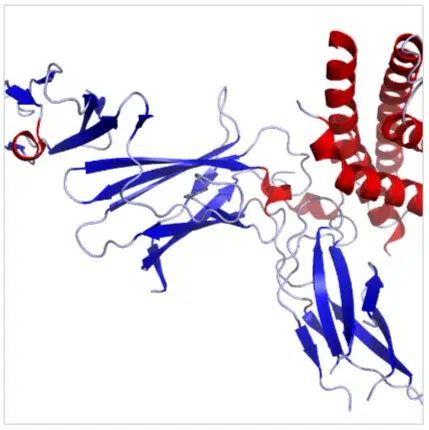
As a reliable vehicle for systemic drug/biologics delivery allowing unique PK/PD capabilities while remaining fully biocompatible with no signs of delayed toxicity from the downstream polymer’s breakdown molecules
Suspension of nanoparticles with a particle size distribution of 100nm to 1000nm
The final product of PLGA encapsulated IL-12 having a range of particle size from 100nm-1000nm, zeta potentials less than -30 mV, encapsulation efficiency between 20-40%, and with an average elution of 1ng of IL-12/1 gram of particles/day.
PLGA Nanoparticles®
PLGA Nanoparticles Pre-Clinical
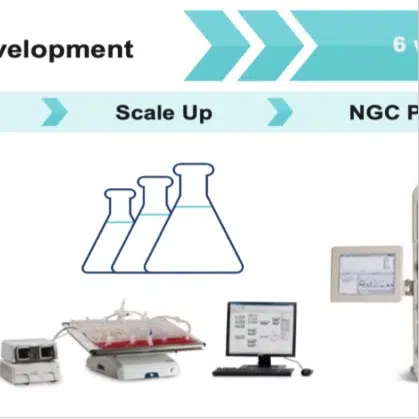
As a reliable vehicle for systemic drug/biologics delivery allowing unique PK/PD capabilities while remaining fully biocompatible with no signs of delayed toxicity from the downstream polymer’s breakdown molecules
Suspension of nanoparticles with a particle size distribution of 100nm to 1000nm
The final product of PLGA encapsulated IL-12 having a range of particle size from 100nm-1000nm, zeta potentials less than -30 mV, encapsulation efficiency between 20-40%, and with an average elution of 1ng of IL-12/1 gram of particles/day.
rHUIL-12/PLGA nanoparticles
rHulL-12/PLGA Pre-Clinical
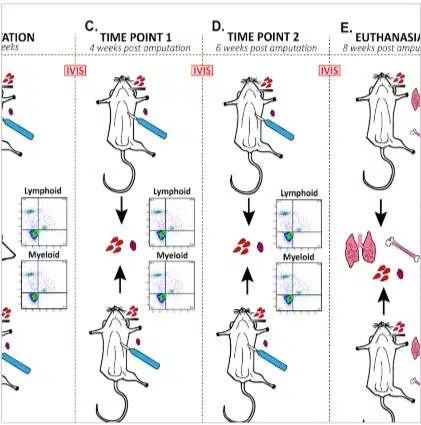
As a reliable vehicle for systemic drug/biologics delivery allowing unique PK/PD capabilities while remaining fully biocompatible with no signs of delayed toxicity from the downstream polymer’s breakdown molecules
Suspension of nanoparticles with a particle size distribution of 100nm to 1000nm
The final product of PLGA encapsulated IL-12 having a range of particle size from 100nm-1000nm, zeta potentials less than -30 mV, encapsulation efficiency between 20-40%, and with an average elution of 1ng of IL-12/1 gram of particles/day.
Diagnostics
Diagnostics Pre-Clinical
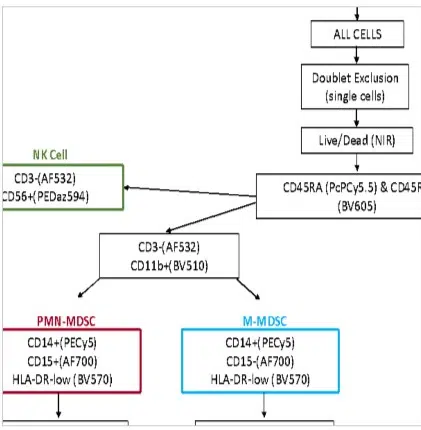
As a reliable vehicle for systemic drug/biologics delivery allowing unique PK/PD capabilities while remaining fully biocompatible with no signs of delayed toxicity from the downstream polymer’s breakdown molecules
Suspension of nanoparticles with a particle size distribution of 100nm to 1000nm
The final product of PLGA encapsulated IL-12 having a range of particle size from 100nm-1000nm, zeta potentials less than -30 mV, encapsulation efficiency between 20-40%, and with an average elution of 1ng of IL-12/1 gram of particles/day.
