Welcome to ICaPath /EYE-SEE-A-PATH/
Pioneering Therapies for the most important people in our lives
Pioneering Therapies for the most important people in our lives
Pioneering Therapies for the most important people in our lives
ICaPath has developed and been awarded an international patent portfolio for its drug delivery technology that combines PLGA nanoparticles with cytokines as powerful immune stimulants. This breakthrough technology has allowed the Company to deliver cytokines in small doses that can uniquely eliminate formidable toxicity issues while achieving extended release in a highly controlled and personalized manner that secures sustained immune efficacy. This novel approach and technologies could be used to meet significant unmet medical needs.
- Non-specific Immunotherapies
- Interleukins (e.g., IL-12), Interferons, Immunomodulators (IMiDs)
- Monoclonal antibodies and immune checkpoint inhibitors
- Naked mAb, Conjugated mAb, Biospecific mAb
- PD-1, PD-L1, CTL-4 inhibitor, TF inhibitor
- Oncolytic virus therapy
- T-cell therapy
- TIL, Engineered TCR, CART-cell, NK-cell therapy
- Cancer vaccines
- Cervarix, Gardasil, HBV, Sipuleucel-T, T-VEC, BCG
- Non-specific Immunotherapies
- Interleukins (e.g., IL-12), Interferons, Immunomodulators (IMiDs)
- Monoclonal antibodies and immune checkpoint inhibitors
- Naked mAb, Conjugated mAb, Biospecific mAb
- PD-1, PD-L1, CTL-4 inhibitor, TF inhibitor
- Oncolytic virus therapy
- T-cell therapy
- TIL, Engineered TCR, CART-cell, NK-cell therapy
- Cancer vaccines
- Cervarix, Gardasil, HBV, Sipuleucel-T, T-VEC, BCG
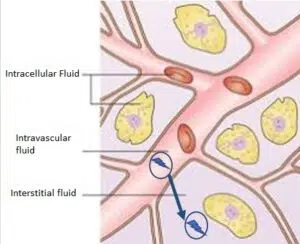
PLGA nanoparticle move from intra to extra vascular (EV) space
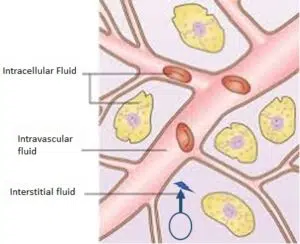
PLGA nanoparticle releases rHu-IL12 into EV space
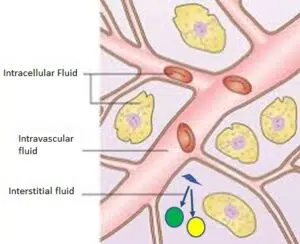
rHU-IL12 activates T and NK cells to recognize/destroy tumor

PLGA nanoparticle move from intra to extra vascular (EV) space

PLGA nanoparticle releases rHu-IL12 into EV space

rHU-IL12 activates T and NK cells to recognize/destroy tumor
INNOVATION
What Sets ICaPath apart from the Rest
What Sets ICaPath apart from the Rest
What Sets ICaPath apart from the Rest
ICaPath’s PLGA nanoparticle-based therapeutic and delivery systems has outperformed traditional immunotherapies in pre-clinical studies with several key areas:
- Enhanced Stability and Controlled Release: This enhances the therapeutic efficacy by maintaining consistent drug levels over an extended period, which is critical for the effective treatment of chronic conditions like cancer.
- Improved Biodistribution and Immune Friendly: PLGA nanoparticles can be engineered to optimize biodistribution, ensuring that the therapeutic agents reach tissues while minimizing off-target effects and reducing systemic toxicity.
- Overcoming Limitations of Direct Competitor Therapeutics:
| ✔ Liposome delivery can often suffer with stability issues and variable drug release profiles, of which our PLGA nanospheres do not. | ||
| ✔ Pegylation may prolong the half-life of cytokines but often at the cost of reduced bioactivity, whereas PLGA nanoparticles avoid this by providing sustained release without altering the bioactivity of the encapsulated cytokine. | ||
| ✔ Immunocytokines traditionally face challenges in resistance with targeted moiety, production complexity and immunogenicity, versus ours that provides precise cytokine release, thus its efficacy. |
ICaPath’s PLGA nanoparticle-based therapeutic and delivery systems has outperformed traditional immunotherapies in pre-clinical studies with several key areas:
- Enhanced Stability and Controlled Release: This enhances the therapeutic efficacy by maintaining consistent drug levels over an extended period, which is critical for the effective treatment of chronic conditions like cancer.
- Improved Biodistribution and Immune Friendly: PLGA nanoparticles can be engineered to optimize biodistribution, ensuring that the therapeutic agents reach tissues while minimizing off-target effects and reducing systemic toxicity.
- Overcoming Limitations of Direct Competitor Therapeutics:
| ✔ Liposome delivery can often suffer with stability issues and variable drug release profiles, of which our PLGA nanospheres do not. | ||
| ✔ Pegylation may prolong the half-life of cytokines but often at the cost of reduced bioactivity, whereas PLGA nanoparticles avoid this by providing sustained release without altering the bioactivity of the encapsulated cytokine. | ||
| ✔ Immunocytokines traditionally face challenges in resistance with targeted moiety, production complexity and immunogenicity, versus ours that provides precise cytokine release, thus its efficacy. |
RESEARCH & TECHNOLOGY
Unique Intellectual Property and Portfolio
Unique Intellectual Property and Portfolio
Unique Intellectual Property and Portfolio
• Awarded Global Patent (Aug 2024) for PLGA nanoparticle technology
• Represents a breakthrough in biologics delivery
✔ Overcomes fragility associated with biologics
✔ Enhances pharmacokinetic properties
✔ Opportunities for licensing deals
- Awarded Global Patent (Aug 2024) for PLGA nanoparticle technology
- Represents a breakthrough in biologics delivery
✔ Overcomes fragility associated with biologics
✔ Enhances pharmacokinetic properties
✔ Opportunities for licensing deals
Introduction to our projects
rHul L-12
- IL-12 is a strong stimulator of both innate and adaptive immunity via T-cells and natural killer cells that has shown great efficacy against pleomorphic neoplastic diseases (e.g., osteosarcomas)
- IL-12 has shown major toxicity and undesirable adverse events when utilized systemically and unencapsulated in initially much higher doses
- IL-12 broad immunostimulation allows the human immune system to react, adapt, and mount effective cell and antibody mediated cytotoxic responses against fast growing neoplastic cells while blocking the development of cancer-driven angiogenesis
PLGA
- Proprietary PLGA nanoparticle technology capable of providing efficient delivery options for companies working with drugs and biologics, including genetic constructs currently being utilized in breakthrough vaccines
- The final product of PLGA encapsulated IL-12 having a range of particle size from 100nm-1000nm, zeta potentials less than -30 mV, encapsulation efficiency between 20-40%, and with an average elution of 1ng of IL-12/1 gram of particles/day.
lL-12 + PLGA
- The systemic use of IL-12 has major and potentially lethal toxicity (Lee et al., 1998).
- systemic flu-like symptoms (fever, chills, fatigue, arthromyalgia, headache)
- bone marrow toxicity leading to important hematologic complications (neutropenia and thrombocytopenia)
- hepatotoxicity leading to direct hepatic dysfunction manifested mainly and dose-dependently by significant hepatocellular necrosis
- systemic effects characterized by undesirable inflammation in their mucus membranes (e.g., oral mucositis, stomatitis, or colitis).
Historically, free non-encapsulated IL-12 was developed and utilized under a MTD paradigm that was common decades ago. However, this approach led to one of two extreme immune pathways, with potentially severe and even fatal outcomes. The immune consequences of high dose therapy from prior development programs were:
- Overstimulation of the immune system leading to systemic inflammatory response syndrome (SIRS), characterized by tachycardia, tachypnea, hypo/hyperthermia, and leukocytosis/leukopenia.
- Immune exhaustion, leading to a further immunocompromised state
Diagnostic
- Fluorescently labeling both surface and intracellular components of isolated human leukocytes allows the real-time analysis of immune status using flow cytometry. methods include Live/Dead staining, blocking of Fc receptors, epitope-specific cell-surface staining, cell fixation and permeabilization, and epitope-specific intracellular staining; staining is via fluorescently conjugated antibodies and amine reactive dyes. Methods described enable downstream analysis for the identification of live cells that are subsequently categorized by the presence or absence of the cellular epitopes and expression markers CD45RA, CD45RO, CD56, CD11b, cd14, cd15, Lox-1, HLA-DR, CD3, CD4, CD8, CD25, Fox-P3, CD127, CTLA-4, Pd-L1, PD-1, and Tim-3.
rHul L-12
- IL-12 is a strong stimulator of both innate and adaptive immunity via T-cells and natural killer cells that has shown great efficacy against pleomorphic neoplastic diseases (e.g., osteosarcomas)
- IL-12 has shown major toxicity and undesirable adverse events when utilized systemically and unencapsulated in initially much higher doses
- IL-12 broad immunostimulation allows the human immune system to react, adapt, and mount effective cell and antibody mediated cytotoxic responses against fast growing neoplastic cells while blocking the development of cancer-driven angiogenesis
PLGA
- Proprietary PLGA nanoparticle technology capable of providing efficient delivery options for companies working with drugs and biologics, including genetic constructs currently being utilized in breakthrough vaccines
- The final product of PLGA encapsulated IL-12 having a range of particle size from 100nm-1000nm, zeta potentials less than -30 mV, encapsulation efficiency between 20-40%, and with an average elution of 1ng of IL-12/1 gram of particles/day.
lL-12 + PLGA
- The systemic use of IL-12 has major and potentially lethal toxicity (Lee et al., 1998).
- systemic flu-like symptoms (fever, chills, fatigue, arthromyalgia, headache)
- bone marrow toxicity leading to important hematologic complications (neutropenia and thrombocytopenia)
- hepatotoxicity leading to direct hepatic dysfunction manifested mainly and dose-dependently by significant hepatocellular necrosis
- systemic effects characterized by undesirable inflammation in their mucus membranes (e.g., oral mucositis, stomatitis, or colitis).
Historically, free non-encapsulated IL-12 was developed and utilized under a MTD paradigm that was common decades ago. However, this approach led to one of two extreme immune pathways, with potentially severe and even fatal outcomes. The immune consequences of high dose therapy from prior development programs were:
- Overstimulation of the immune system leading to systemic inflammatory response syndrome (SIRS), characterized by tachycardia, tachypnea, hypo/hyperthermia, and leukocytosis/leukopenia.
- Immune exhaustion, leading to a further immunocompromised state
Diagnostic
- Fluorescently labeling both surface and intracellular components of isolated human leukocytes allows the real-time analysis of immune status using flow cytometry. methods include Live/Dead staining, blocking of Fc receptors, epitope-specific cell-surface staining, cell fixation and permeabilization, and epitope-specific intracellular staining; staining is via fluorescently conjugated antibodies and amine reactive dyes. Methods described enable downstream analysis for the identification of live cells that are subsequently categorized by the presence or absence of the cellular epitopes and expression markers CD45RA, CD45RO, CD56, CD11b, cd14, cd15, Lox-1, HLA-DR, CD3, CD4, CD8, CD25, Fox-P3, CD127, CTLA-4, Pd-L1, PD-1, and Tim-3.
PIPELINE
We are developing a broad portfolio of products designed to cure serious diseases in new ways
We are developing a broad portfolio of products designed to cure serious diseases in new ways
We are developing a broad portfolio of products designed to cure serious diseases in new ways
Technology
Preclinical
Clinical
Phase 1
Phase 2
Phase 3
approval
IL12 Protein
PLGA
IL12+PLGA
Diagnostic
Our Current Research
ICaPath is currently manufacturing rHu IL-12 and PLGA nanoparticles under GMP guidelines in 2 world-class CDMOs to support the upcoming phase first in human clinical trial
Dedicated to the improvement of lives
Dedicated to the improvement of lives
Dedicated to the improvement of lives
From our founder
ICaPath has spent over a decade to pioneer the use of prolonged and very low dose IL-12 encapsulated into a new delivery PLGA nanoparticle platform while providing a new diagnostic test to provide continuous immunosurveillance for patients undergoing immunotherapy.
ICaPath has spent over a decade to pioneer the use of prolonged and very low dose IL-12 encapsulated into a new delivery PLGA nanoparticle platform while providing a new diagnostic test to provide continuous immunosurveillance for patients undergoing immunotherapy.
BROCK LINDSEY MD
BROCK LINDSEY MD
Safe delivery, precise and adaptable dosing and assuring efficacy are our main goals.
Our Team
Our Team
ICaPath is made up of members and researchers located across the North Eastern region of the United States. We have assembled a team of the most qualified people to work with us, regardless of location. Our practice locations include some of the nations’ leading healthcare facilities.

Founder, Chairman of the Board, CSO
BROCK LINDSEY MD
BROCK LINDSEY MD
Associate Professor Department of Orthopaedic Surgery Johns Hopkins University

Executive Vice Chairman
Robert G. Finizio
Robert G. Finizio
Executive Director at Pleo Pharma
General Partner with MintPharma Capital
Board of Directors for PleoPharma, Myosin Therapeutics, Vycellix, NOXX Therapeitics and Zyversa Therapeutics
Chairman Emeritus of Bio-Florida
Board Member for the Palm Beach County Sheriff’s Office Foundation
Co-Founder TherapeuticsMD
Co-Founder CareFusion

PRESIDENT
Steve Norch
Steve Norch
Former CEO Biomath Solutions
Former CEO EK Norch Inc
CEO Ocius Intelligence

Chief Medical Officer, and CMC
PAULO FONTES, MD, FACS
PAULO FONTES, MD, FACS
Chief Scientific/Medical Officer, Eikonoklastes Inc., Cincinnati, OH
Co-Founder & Medical Advisor LyGenesis, Pittsburgh, PA
Co-Founder, VirTech Bio, Natick, MA
Former Professor of Surgery & Deputy Director, McGowan Institute for Regenerative Medicine, School of Medicine, University of Pittsburgh

Chief Scientific Advisor
James S. Allan, M.D.
James S. Allan, M.D.
Thoracic Surgery at Massachusetts General Hospital
Chief of Thoracic Surgery at Wentworth-Douglass Hospital
Associate Chief of Thoracic Surgery at Salem Hospital
Co-Director of the Cardiothoracic Transplantation Laboratory in MGH’s Center for Transplantation Sciences
Chair of the MGH Institutional Animal Care and Use Committee
Past-President of the American Society of Transplantation
Chair of the Board of Directors of the Foundation for Biomedical Research
President of the Foundation for Research, Education and Community Engagement
Board Of Directors
Board Of Directors
ICaPath’s Board of Directors plays a key role in guiding our early-stage growth with deep expertise in biotech, capital markets, and strategic oversight. Focused on ethical governance and long-term value creation, the Board supports our mission to advance breakthrough cancer therapies and helps position the company for successful clinical and commercial milestones.

Board Member
Tim McInerney
Tim McInerney
Board Member of Corino Therapeutics
Former Chairman of Insite Vision(acquired by Sun Pharmaceutical)
Former Board Member Edgemont Pharmaceuticals (acquired by Alvogen,Inc)
Former Board Member Emisphere Technologies
Paramount Biocapital
Bristol Myers
Bear Stearns
Ladenburg Thalmann

Board Member
David Adams
David Adams
Co-Founder of MintPharma Capital Investment Fund
Managing Partner of Mint12, a Specialized Alternative Investment Management Company
Consultants
FINANCIAL
Tony Wey and Biotech Group, CliftonLarsonAllen (CLA), Minnesota LLP
IP FIRM
Wilson, Sonsini, Goodrich, & Rosati, Boston, MA
GENERAL COUNSEL
Doug Boggs, DLA Piper, Washington DC
FDA CONSULTANT
Clementi & Associates, Rosemont, PA
Investors
Thank you for your interest in ICaPath
Thank you for your interest in ICaPath
Thank you for your interest in ICaPath
At ICaPath, we are pioneering breakthrough therapies designed to revolutionize cancer treatment through our proprietary PLGA nanoparticle platform. Investors are invited to explore this unique opportunity to be part of our mission to advance potentially life-saving technologies.
For more detailed insights into our strategic roadmap and future growth potential, please review our Executive Summary and Investor Presentation.
Discover how ICaPath is positioned to create a lasting impact in the biotech industry, driving innovation and returns.
Get in Touch
Get in Touch
We take pride in the work we do, and are always willing to talk about the impact our research will have. For more information, or to discuss collaboration or investments please reach out.
We take pride in the work we do, and are always willing to talk about the impact our research will have. For more information, or to discuss collaboration or investments please reach out.

